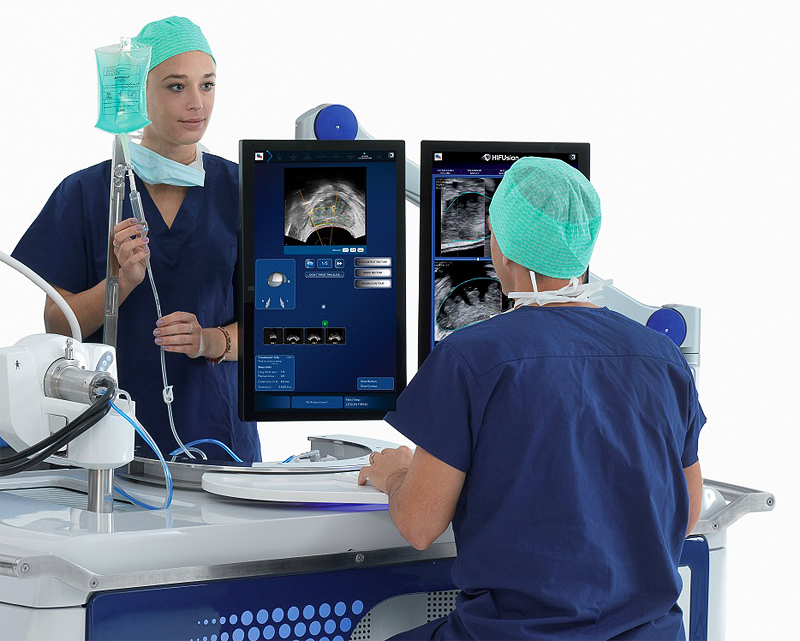
In February 2019 Saint John’s Cancer Institute (formerly John Wayne Cancer Institute) at the Providence Saint John’s Health Center became the first medical center in the U.S. to acquire Focal One high intensity focused ultrasound (HIFU) for treating patients with localized prostate cancer. Focal One is one of the most precise tools available for delivering targeted, non-invasive ablation of diseased prostate tissue.
Saint John’s Cancer Institute at Providence Saint John’s has received worldwide acclaim for advances in cancer treatment and research, including research in prostate and liver cancer. The institute’s unique ability to rapidly turn scientific breakthroughs into innovative approaches to treatment and early detection provides immediate hope to cancer patients from around the world.

1. Can you talk a little about the Focal One technology, and describe what makes this ultrasound technology the world’s most advanced prostate ablation device?
Focal One, manufactured by EDAP TMS is the newest generation high intensity focused ultrasound (HIFU) system which received FDA clearance in June 2018. Focal One fuses high resolution magnetic resonance images with biopsy results and real-time ultrasound images — a unique collaboration between radiology, pathology and urology.
Using Focal One, the urologist can view these integrated, detailed 3D images of the prostate on a large monitor, then draw precise contours around the diseased tissue and ablate only that portion of the prostate. This enables the treatment of only the areas of the prostate harboring cancer, as seen on the MRI, while leaving healthy tissue intact. With Focal One, the urologist can customize the procedure for each patient and each clinical condition. These advances in ablation technology complement at Providence Saint John’s experience with numerous minimally invasive treatment devices, leading the nation in innovative prostate cancer treatments.
2. In a similar vein, can you talk about the impact that this device could have on the treatment of prostate cancer, and how it differs from current treatment methods?
There are basically 3 ways to manage prostate cancer. The first is watchful waiting or active surveillance. Prostate cancer is slow growing and may not impact the patient during his lifetime, especially when considering that most prostate cancers are not aggressive. Despite reassuring data in large randomized trials, some patients are still uncomfortable with this choice of management and prefer treatment. In this group, treatment has to be weighed against treatment side-effects, since many will never die of their disease.
On the other end of spectrum, we have complete treatment of the prostate, which involves either surgery (radical prostatectomy) or radiation therapy. Although recent innovations like robotic surgery and newer radiation devices have improved the technology of these treatments, side-effects that change the normal function of the bowel, bladder, and erections still exist.
HIFU – and now Focal One — fills a significant void between active surveillance and radical treatments. HIFU uses high-frequency sound waves directed at the cancerous tissue through an ultrasound probe inserted into the rectum. The high intensity sound waves heat and destroy the targeted tissue, causing cell death. With HIFU you are able to destroy the cancerous tissue without damaging other surrounding structures, which include nerves, blood vessels and muscle tissue that is important in erections and maintaining continence of urine.
This is similar to a lumpectomy where the surgeon removes only the breast cancer tumor vs. the entire breast, urologists can now use Focal One for HIFU focal therapy to target the exact location of the cancerous tissue and destroy it, and spare the rest of the normal prostate, leading to less side-effects.
3. What kinds of results have you seen with this technology thus far, either in clinical studies or in early roll out stages? How encouraging have the results been so far?
More than 45,000 men around the world with localized prostate cancer have gone through the HIFU procedure. In Europe, where the first generation HIFU system originated and was used for whole prostate treatment, researchers published positive peer-reviewed studies of the use of HIFU for whole gland ablation.
In 2013, the Journal of Urology published a German study of 704 patients treated with HIFU, in which co-investigators concluded that long-term follow-up with HIFU therapy demonstrated a high overall rate of cancer specific survival (99%) and 10-year salvage treatment-free (meaning these patients did not require additional treatment for cancer after 10 years) rates of 98% in low-risk, 72% in intermediate-risk, and 68% in high-risk patients.
One must still understand that focal therapy is a very new treatment option, especially with this particular ablation device, the Focal One, only receiving market clearance in June of 2018. Long-term studies and clinical data are not yet available and this therapy is only appropriate for select prostate cancers. However, the arrival of the Focal One by EDAP TMS is significant because of the degree of precision with which the cancer can be treated in the prostate; sparing healthy tissue and reducing side-effects of treatment.
4. Are there any drawbacks or potential risks involved in using this technology or taking this approach to treating prostate cancer?
As discussed earlier, although focal HIFU treatment is available for localized prostate cancer and studies in Europe have demonstrated its safety and efficacy, there are no long term follow up data in the US at this time. Urologic surgeons in the US will need at least 10 years of data to establish focal therapy as a standard treatment.
However, with HIFU, if the patient’s cancer returns he can undergo radical surgery or radiation, or repeat HIFU. In other words, HIFU does not preclude a secondary treatment, whereas the two conventional treatments, if unsuccessful, cannot be repeated, and secondary treatment options are limited and come with a risk of debilitating side effects.
5. Finally, with the news that this device will be used at UCI Health, Saint John’s Cancer Institute, Houston Methodist Hospital, and Maimondies Medical Center in Brookly, how soon do you think we’ll begin to see this technology being adopted as a regular treatment method for prostate cancer? What, if anything, needs to happen to help bring that reality to fruition?
The fact that four renowned medical centers acquired Focal One within two months of each other shows the growing interest in Focal One from many highly-regarded healthcare institutions across the U.S. It’s also a testament to the potential clinical advantages of Focal One compared to other currently available prostate cancer treatments.


14 Replies to “Focal One FAQs – High Intensity Focused Ultrasound for Treating Prostate Cancer”
Michael Grand
Hello,
I was wondering if you have had a chance to perform a procedure using the equipment and if so what is your initial thoughts on the procedure (as I have heard there is a high learning curve) and being one of the only west coast owners of the machine have you trained any other staff from different hospitals on it? I’m hoping soon insurance will cover the procedure in the near future so that it can be very accessible.
Thank you so much for the article.
fahdoot
Thank you for the comment Michael. We are scheduling our first patients as we speak and based on what we already saw at European expert sites during our initial training, we believe the learning curve is not long.
Michael Grand
Hello,
Sorry to bother you but I was wondering if you have an update since the last comment I’ve made. I’m excited to hear of the progress HIFU is making and can’t wait to hear about the initial patients.
Thank you
Fariba Ahdoot
Hi Michael,
We are pleased to announce our first patients were successfully treated and doing fine. The procedures went very well with the support of an international expert. We are comfortable with the device and the procedure to treat more patients and become a reference center for Focal One in California.
Michael Grand
Hello,
Sorry to send you so many messages, I appreciate the time you are taking out of your busy schedule and I promise this will be the last. I understand you want to become the reference center for Focal One in California and just wanted to know if doctors in the area have shown interest or if you have had the chance to teach any of the doctors in the area on Focal One. I’m trying to get a feel for how much interest HIFU is getting in the area as I hope it is building momentum in the US.
Thank you so much
Fariba Ahdoot
Hi Michael,
Thank you for your comment. Our blog is really geared toward patients, caregivers and the community. We hope people will learn more about Focal One and consider it as an option for prostate cancer treatment, if they are eligible.
rachel frampton
My father has been diagnosed with prostate cancer and we’re still looking for treatments that can help him. It’s surprising to learn that prostate cancer is slow-growing and may not impact the patient right away. I hope we’ll be able to find a great treatment for my dad.
Fariba Ahdoot
Thank you for your comment Rachel. We’re sorry to hear about your father’s diagnosis. We offer a variety of treatment options for prostate cancer including Focal One: non-invasive robotic-assisted treatment, chemotherapy, radiation therapy and hormone therapy. Please contact us to schedule an appointment for your father to discuss which personalized treatment option is best for him by using the link below: https://www.saintjohnscancer.org/contact-us/?center=Urology%20and%20Urologic%20Oncology.
Paul R
I have early stage Prostate cancer with only one sample < 5% and Gleason score of 3+3
The MRI was not able to see a tumor.
Am I a candidate for this new MRI/Ultrasound/Biopsy integrated technology if the MRI has not shown a tumor?
Thank you very much
Paul
Stephen McNally
Hello,
I was recently diagnosed as Stage 1, prostate cancer, with a gleason score of 7, 4+3.
I live a portion of the year in Mexico, and have access to the sonablate technology, which sounds very promising. I was told that I would be an ideal candidate for the procedure. The cost is a bit prohibitive at $15,000 USD out of pocket, but one can’t put a price on good health and a good life.
Are you aware of any ongoing or upcoming clinical trials of which I could apply for?
Thank you for your time and consideration.
Stephen McNally
Fariba Ahdoot
Hi Paul,
Thank you for your comment. Please call our Urology and Urologic Oncology Center at 310-582-7137 or fill out our online form linked below to speak with our experts. https://www.saintjohnscancer.org/contact-us/?center=Urology%20and%20Urologic%20Oncology
Thank you!
Fariba Ahdoot
Hi Stephen,
Thank you for your comment. Please call our Urology and Urologic Oncology Center at 310-582-7137 or fill out our online form linked below to speak with our experts. https://www.saintjohnscancer.org/contact-us/?center=Urology%20and%20Urologic%20Oncology
You can also view our current clinical trails here: https://www.saintjohnscancer.org/clinical-trials/
Thank you!
Ken Viprino
Recently diagnosed stage 1 Gleason # 7 4+3 I was told I may not be a candidate as my cancer is in 2 sides of prostate Any thoughts ?!
Fariba Ahdoot
Hi Ken,
You can contact our Urology and Urologic Oncology Center at 310-582-7137, to schedule an appointment and discuss your eligibility.
Thank you!
Comments are closed.