Introduction to Drug Repurposing for Rare Diseases
Although rare diseases affect a proportionally smaller number of patients, they represent a compelling unmet medical need as many of them lack effective treatments. The drug discovery and development process is long and expensive, and can take years to deliver new treatments to patients. A possible alternative to this process is to repurpose pre-existing, approved drugs for different diseases, bypassing conventional slow-moving drug discovery. Through the National Cancer Institute’s Department of Therapeutics Program, we have access to a diverse library of pre-approved compound sets.
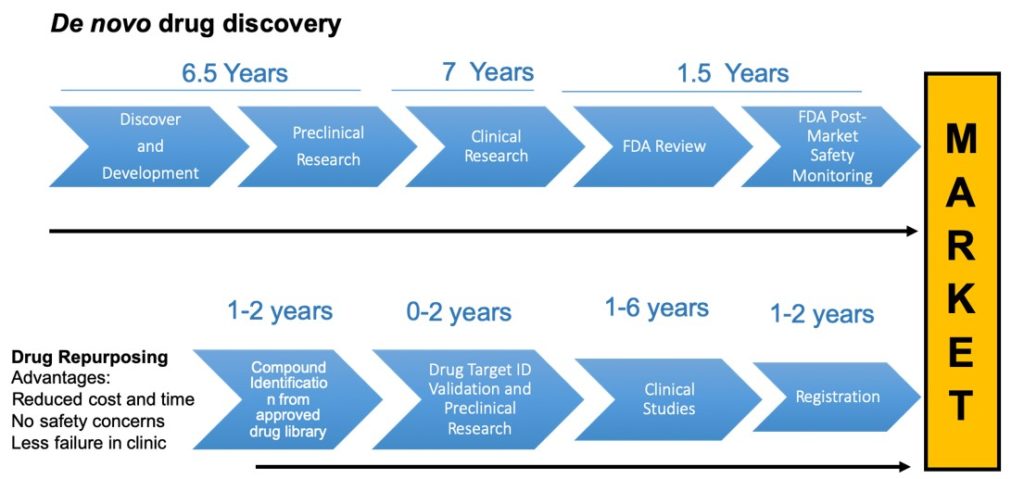
These compounds have accumulated preclinical and clinical data that can be utilized to make the process of further development much faster and more efficient. Our goal is to accelerate the development of new treatments for rare diseases by screening these compound library sets for new therapeutic applications. We apply new technologies and approaches for screening, such as phenotypic cell based disease models and high throughput screening (HTS).
Disease models:
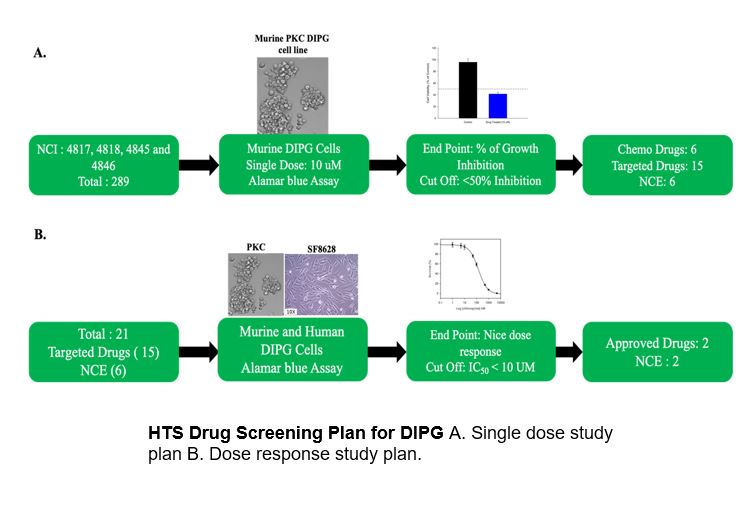
Diffuse Intrinsic Pontine Glioma