Our Approach
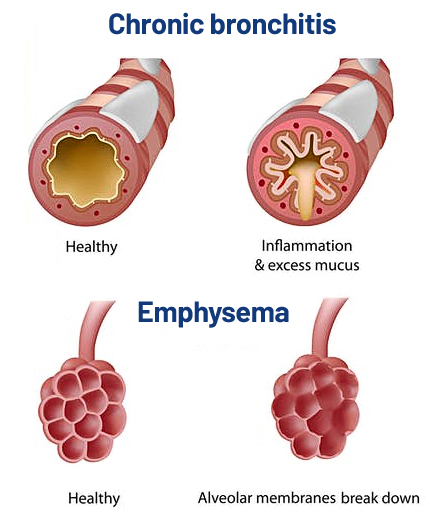
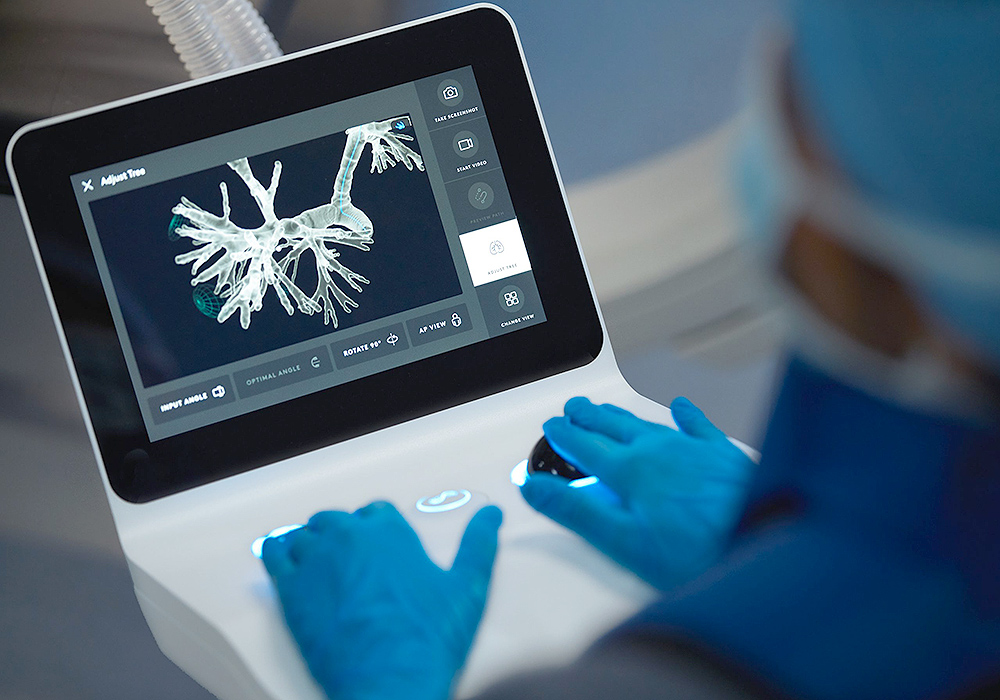
Our commitment is to care for you. As a global leader in thoracic and lung surgery we continue to improve our patient’s quality of life. By utilizing minimally invasive treatment options and advancements in technology, including robot-assisted surgery, transitioning back to normal activities is easier for patients, with shorter recovery time, and fewer side effects.
“We are a comprehensive, multidisciplinary Thoracic Center of Excellence that is ready to support you.”